“It is important to realize that in physics today, we have no knowledge of what energy is. We do not have a picture that energy comes in little blobs of a definite amount.”
[1]- Richard Feynman
We often think of energy as some universally defined quantity with a discrete unit of measurement.
For example, we can say that a particle “has” energy.
This makes it sound like there’s some independent property of the particle called “energy” that it possesses, just like it has a position and a mass.
Looking at energy from this lens can be useful for practical calculations.
But it obscures the simplicity of what energy really is.
If we start from a few basic observations about the universe, we can arrive at much more satisfying and intuitive understanding of energy.
Energy
In our universe, things change.
At a large scale, there seems to be a diversity of ways that things can change.
But at a small scale, all observable change comes down to changes in the movement of particles.
We say a ball is rolling when all the particles in the ball start to move in unison.
We say the temperature in a room rises when the particles in the room move and collide more rapidly.
Over time, we noticed that there were consistencies in the movement of particles, and the emergent behavior that resulted from it.
These consistencies could all be described with a set of 4 forces: gravity, electromagnetism, strong force, and weak force.
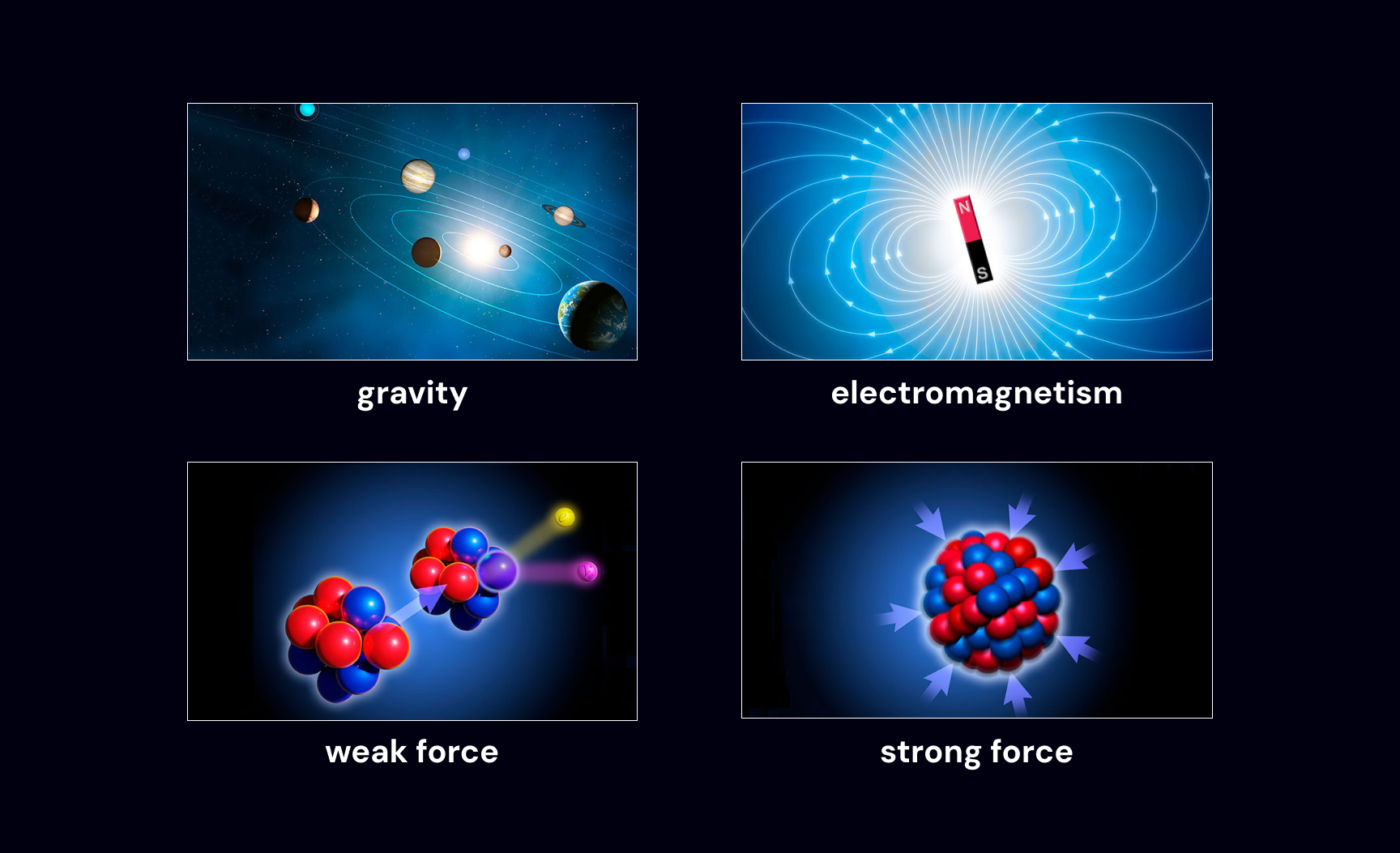
the 4 fundamental forces
All movement is caused by these forces.
They also allow some particles to store the potential to cause movement in the future.
These 2 effects of forces define the only changes that can occur in our universe:
- Changes in the movement of particles
- Changes in the potential to move particles in the future
So particles can cause changes to other particles based on the fundamental forces. This is where energy comes in.
Anything with the ability to cause change in the universe is said to have energy.
Energy is simply the ability to cause change.
Energy exchanges
We often hear that energy constantly changes forms.
This framing is misleading.
What we really mean is that some things in the universe are always causing changes to other things. For example, a moving particle may collide with a stationary particle, causing the stationary particle to start moving, and the original particle to slow down.
When such an interaction happens between two things in the universe, the first thing decreases its ability to cause change, and the second thing increases its ability to cause change.
By our definition, energy has then been passed from the first thing to the second thing in such an exchange.
We can then say that the form of energy refers to the type of thing that's carrying the energy.
So when we say that energy constantly changes forms, we mean that the types of things that are carrying energy are changing.
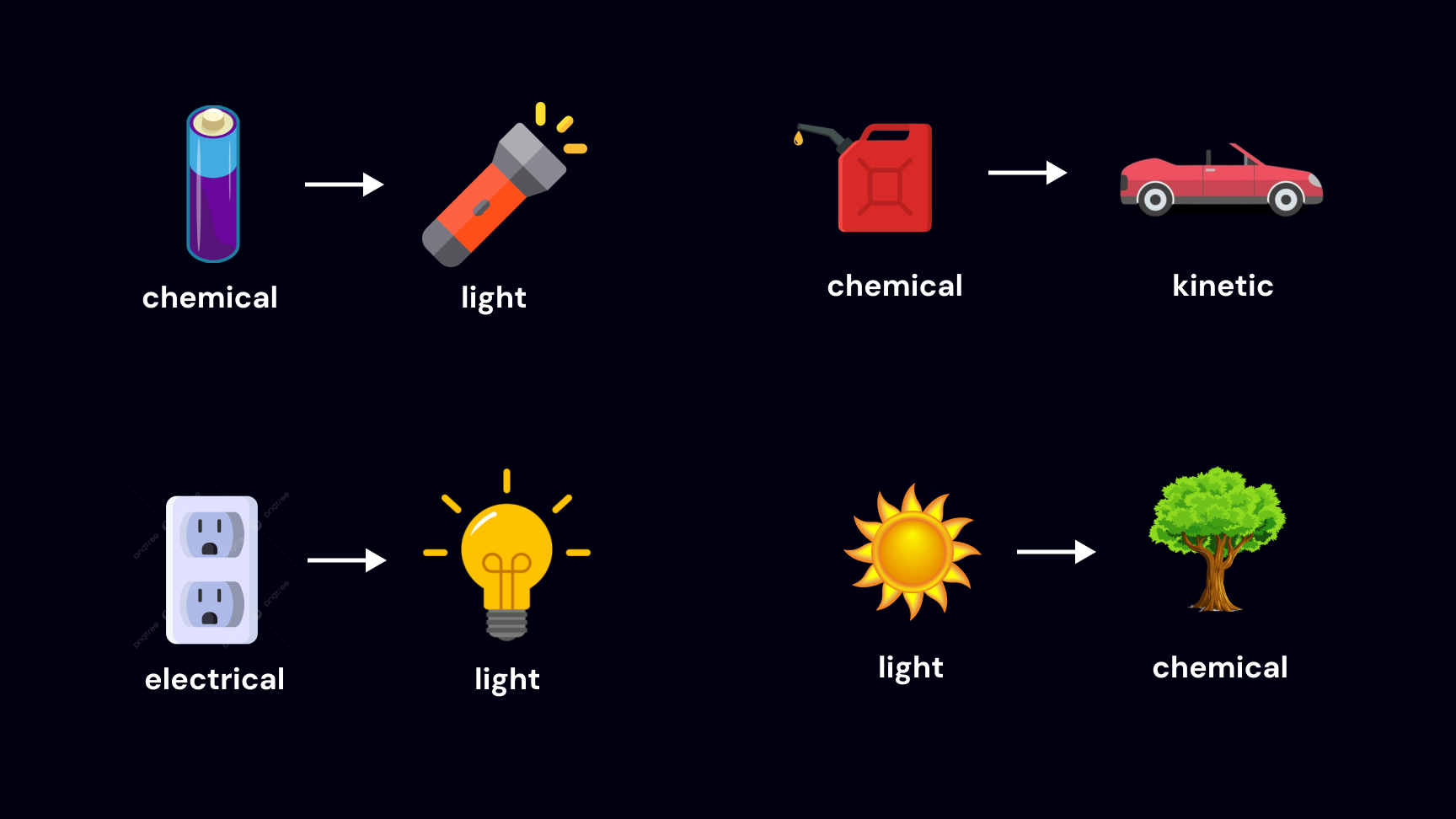
common energy exchanges
Then, we can redefine some common forms of energy you may have heard of with this distinction:
- Kinetic Energy - The energy held by a moving particle
- Thermal Energy - The energy held by many moving particles in an enclosed space that are colliding with each other; really the aggregate effect of kinetic energy
- Radiant Energy - The energy held by a photon
- Gravitational Energy - The energy held by a mass based on its position relative to another mass
- Electromagnetic Energy - The energy held by a charge based on its position relative to another charge
- Nuclear Energy - The energy held within the nucleus of an atom; the effect of the strong and weak nuclear forces

common distinctions of different forms of energy
So all change in the universe involves an exchange of energy between forms.
We can then cause changes in the world by inducing specific energy exchanges that produce desirable results.
This is the principle that our energy systems, and all actions we take in the world, are built on.
We will see that the degree to which we can shape our environments is determined by our ability to control the energy exchanges that occur around us.
Thermodynamics
Thermodynamics is the study of the changes between different forms of energy. It yields 2 critical observations that will influence our broader view on energy.
Conservation of energy
First, the total energy in any closed system is conserved.
This means that the total energy present before and after any energy exchange is equivalent. No new energy is created or destroyed.
At the scale of the universe, no matter what exchanges occur, the total amount of energy remains the same.
This is known as the first law of thermodynamics, or the conservation of energy.
It implies that there’s a finite ability to change stuff in the universe, and in any closed system.
In order to do anything, we need to use some of this finite energy source.
Entropy
Second, the total amount of entropy in any closed system is always increasing.
Put more intuitively, the total amount of useful energy in any closed system is always decreasing.
From the perspective of humanity, not all energy is useful. We only care about forms of energy that we’re capable of using effectively. Energy that’s dispersed isn’t useful to us.
For example, the heat in a room carries energy, but it’s spread out in a way that’s impossible to use for anything practical.
When energy is collected in a small area, we can use it effectively as it's easier to capture and transport. This is why fossil fuels like coal are so useful; they collect a large amount of energy in a small space.
We often say that entropy is a measure of disorder. More directly, entropy is a measure of the lack of usefulness of the energy in a system.
Unfortunately, energy has a natural tendency to spread out over time through diffusion.
Diffusion is often misunderstood. It’s actually the result of probability playing out with random collisions of particles. In any space, particles being spread out is far higher probability.
Increasing dispersion is always more likely, so entropy always increases.
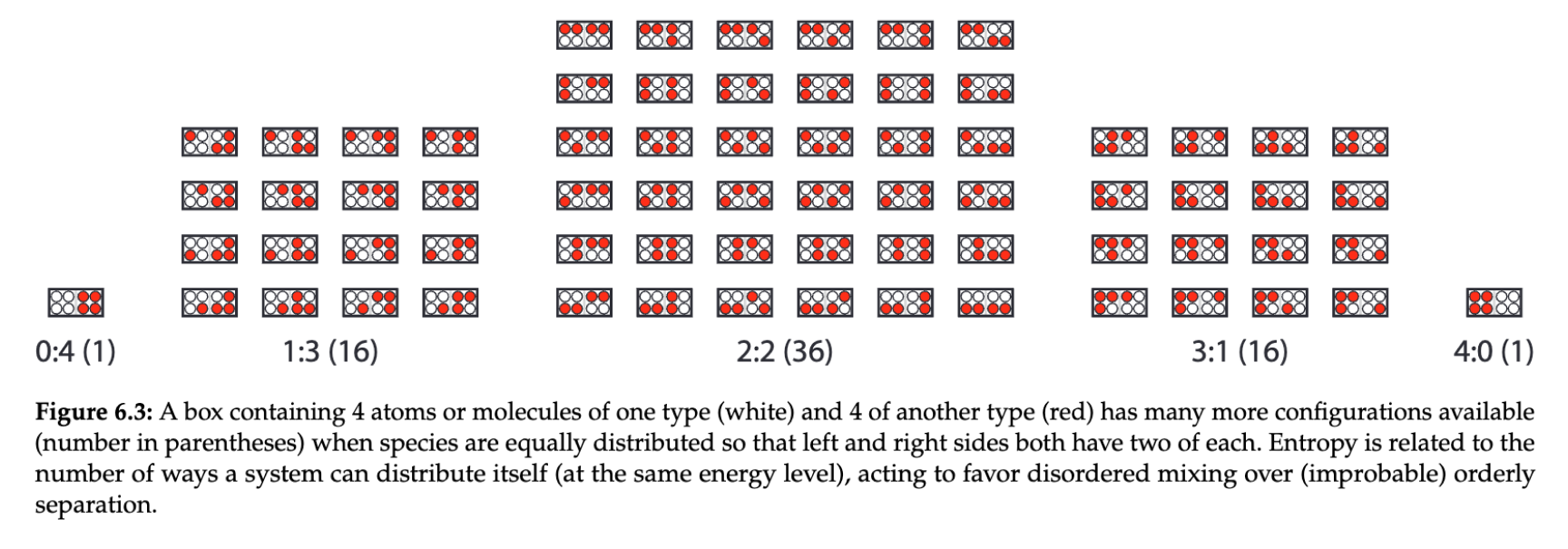
entropy is a natural result of probabilities[2]
In an individual energy exchange, some energy is transferred into a useful form that we can control, but there is always some energy lost to entropy. This energy is lost in the form of heat.
Whenever we use energy, we are increasing entropy.
For example, when we burn coal, we change energy from a low entropy form, stored in condensed coal, into heat which disperses into the atmosphere, and is eventually radiated into space.
We may use some of this heat to create electricity or power a steam engine, but we can never recapture all this heat for use. So the total amount of useful energy in the universe has decreased through this process.
The first law tells us that the total amount of energy in the universe is constant. The second law updates this by telling us that the total amount of available energy is actually decreasing.
Humanity is a thermodynamic incinerator
In our efforts to use more energy, we aren’t just converting energy between different forms. We’re also converting useful energy into useless energy at scale.
In that sense, we’re accelerating the second law of thermodynamics by increasing entropy at a much faster rate than normal.
We do this in order to prevent disorder in our own environments.
At an individual scale, we consume energy to prevent entropy from destroying our bodies. Our bodies take constant energy for cells and larger internal systems to maintain themselves. Without this energy expenditure, we decompose.
At a societal scale we increase entropy by consuming energy, and we use this captured energy to increase order, structure, and growth in our systems.
In order to sustain this constant burning of useful energy, we need a constant energy input into our system from somewhere. And we’re getting that from the sun. As we’ll see in Part III, 100% of the energy we use originates from the sun, or from the remains of ancient stars.
Aside: How did we get here?
You'll see aside boxes throughout this deep dive. These are for interesting tangents that aren't critical to the core narrative.
If entropy is always increasing, then how did we get to the current state of the universe?
We saw that increasing entropy is a result of the universe naturally converging toward more probable states.
This implies that the current state of the universe, with stars, planets, and life on earth, is more probable than the universe right after the Big Bang, which was a mixture of hydrogen and helium particles.
How is this possible? It seems like a mixture of randomly spread out small particles is way more probable than highly condensed matter forming into stars and planets.
Clearly, something is causing certain states of reality to be more probable than others.
In this case, that something is gravity. Because gravity exists, particles being clumped together in larger forms (which eventually make planets and stars) is actually more probable than particles being separated.
We can then view the laws of physics as the primary influences on the probability landscape of reality.
The most probable outcomes are those determined by the 4 fundamental forces.
So the form and structure of our universe today is actually the most likely outcome as defined by physics, and is higher entropy than our past.
Aside: What happens when all the energy is used?
The energy in the universe slowly becomes less useful over time as it disperses.
At some point, the last bit of useful energy will be consumed, and the entropy in the universe will reach it's maximum.
At this point, it will no longer be possible to convert energy to do work. The universe will become uniformly cold and empty. Time will continue, but no further events will occur.
This is known as the heat death of the universe.
Energy & Economic Growth
In every closed system, there’s a finite amount of energy, and a decreasing amount of useful energy. This means the total ability to change stuff in any closed system is decreasing.
This is particularly relevant to us because the earth and the sun make up a roughly closed system, and because the need to change stuff is a core aspect of humanity.
Humans have innate desires. These desires express individuals' wants for realities that don’t yet exist. In order to actualize these realities, change is required.
So at an individual scale, humans naturally desire to enact change.
At a societal scale, markets and economies have emerged in order to efficiently direct these individual desires toward collective abundance.
They have turned humanity into an organism built on constant growth. Modern markets are structured with growth as a fundamental assumption.
So constant growth, which requires constant change, is at the core of our civilization.
This dependence on constant change means our civilization requires ever-increasing amounts of energy to sustain itself.
The amount of economic growth we can achieve is determined by:
- The total amount of energy we consume, which constrains the maximum amount of change we can cause.
- The degree to which we efficiently leverage this energy to enact change.
The advancement of technology is the source of our increasing leverage on energy.
Technology can be seen as the development of new tools that allow us to do more useful stuff with less energy.
To say we are a wealthier society now more than ever before is to say that we have [1] used more energy [2] with better technology [3] to create more abundance.
In this framing, we arrive at Vaclav Smil’s conclusion that the progression of civilization can be viewed as the increasing capture and control of greater amounts of energy, and the more efficient application of that energy to do more useful work.
